CARBOGEN AMCIS expands capacity for parenteral drug manufacturing
In France, on newly acquired land located 7 km from the existing site in Riom north of Clermont-Ferrand in Auvergne, construction have begun in January 2021 with a new, state-of-the-art facility dedicated to custom development and manufacturing of parenteral drug products.
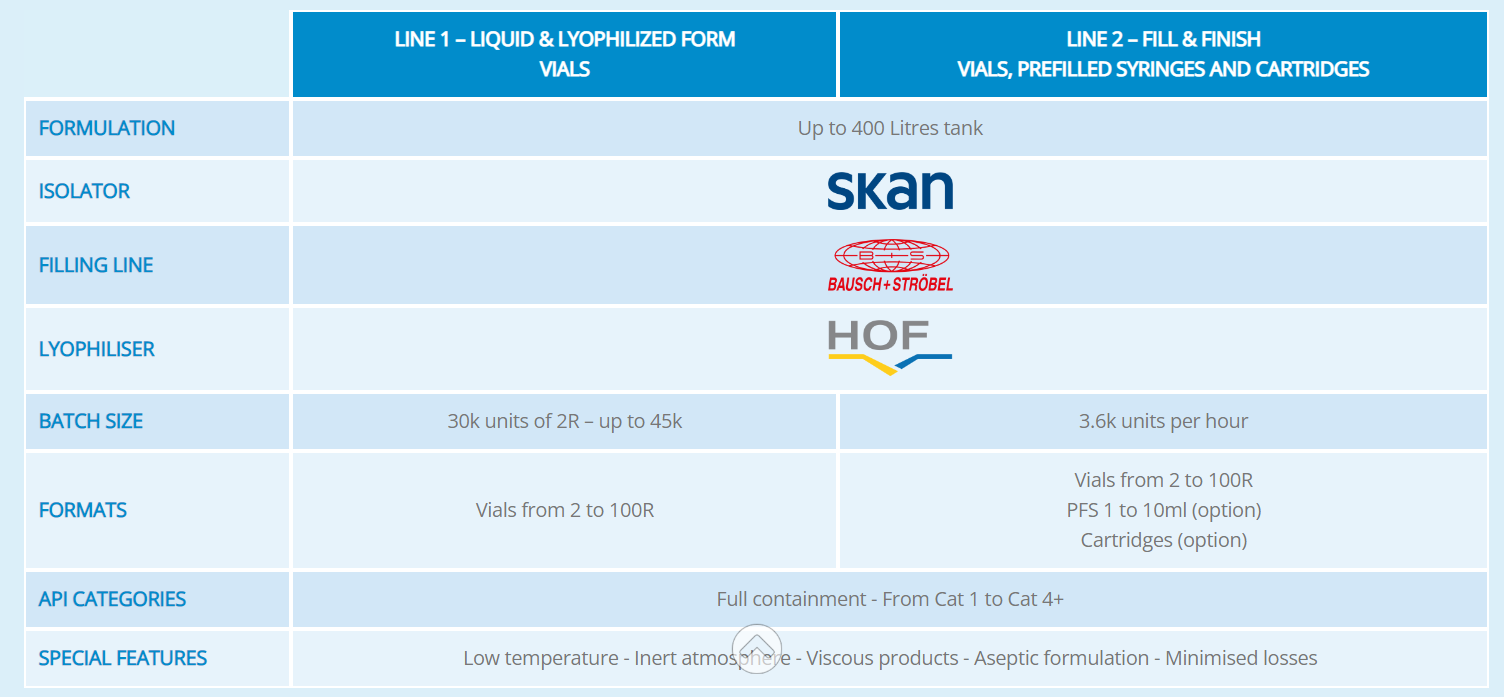
This new state-of-the-art facility will allow for larger capacities to supply our customers with clinical batches for phase III clinical trials and small-scale for commercial. The handling of complex formulations will include a large range of APIs from biologics to highly potent compounds.
Operations will commence during Q1 of 2023.
Teaser Riom 2 - Episode 2: The work is continuing


